If you’ve ever wondered why one medicine comes in a push-through blister, another in a plastic bottle, and a third in a tear-open sachet, the short answer is that different drugs “dislike” different things. Some degrade quickly in moisture, some are sensitive to light or oxygen, some need strict dose control, and some are packaged to reduce misuse or improve day-to-day adherence. This article explains the most common pharmaceutical packaging types in plain English—starting with the three formats people see most (blister packs, bottles, and sachets), then expanding to the other formats you’ll run into, like strip packs, tubes, vials, ampoules, and prefilled syringes.
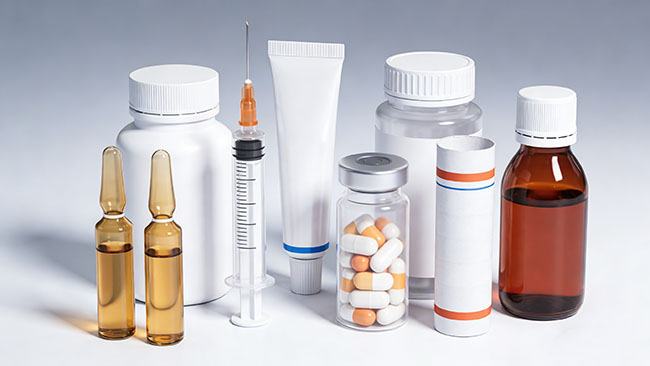
What pharmaceutical packaging really means
When people talk about pharmaceutical packaging types, they’re usually talking about the package that touches the drug (called primary packaging) and the package that protects and labels it for distribution (often called secondary packaging). Primary packaging is the “first line of defense”: it keeps a tablet dry, keeps a cream clean, or keeps an injectable sterile. Secondary packaging is what helps patients and pharmacists handle the medicine safely—think cartons, leaflets, barcodes, and tamper-evidence cues.
For everyday users, the practical way to think about primary packaging is simple: it’s designed to keep the drug stable through shipping and storage, and to deliver a predictable dose when you use it. That’s why a humid bathroom might matter for some tablets, why certain medicines come in darker bottles, and why some doses are separated into calendar packs or unit-dose formats. In other words, pharmaceutical packaging types aren’t just branding—they’re part of drug protection and safe use.
The three formats you see most: blister packs, bottles, and sachets
Blister packs, bottles, and sachets cover a large share of oral medicines and supplements, and they’re the formats most people recognize immediately. Each shines for different reasons, and each has trade-offs that show up in real life—how easy it is to carry, how well it protects against humidity, how visible tampering is, and how consistently people take the right dose.
Blister packs seal each dose into its own cavity. That simple idea explains many of their pros: if you pop one tablet today, the rest stay sealed and protected; and if a pocket is empty, you can see it instantly. Bottles are convenient for larger counts and are often faster to dispense and handle, but once opened they expose all remaining doses to the environment every time the cap comes off. Sachets (and their close cousin, stick packs) are essentially single-serve pouches—great for powders, granules, and drink-mix style products, and very travel-friendly, but they can be less intuitive for some users and often create more individual pieces of waste.
Below is a practical comparison of these three pharmaceutical packaging types. It’s not meant to be “one is best,” but rather “each is best at something.”
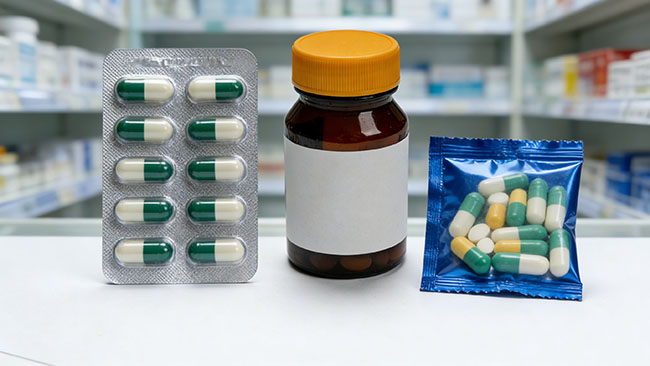
Table 1. Blister vs Bottle vs Sachet (everyday pros and cons)
| Packaging format | Best for | Protection strengths | Everyday convenience | Common trade-offs | Typical production line (behind the pack) |
| Opakowania typu blister | Tablets/capsules needing dose separation | Strong barrier options; dose stays sealed until used | Easy to count doses; visible tamper cues | Some packs can be hard to open; recycling can be tricky | Often produced on a blister packing machine within a blister packaging line |
| Butelki | Higher-count tablets/capsules; broad retail use | Good with desiccant + proper closure; strong logistics | Fast dispensing; familiar | Entire contents exposed after opening; mix-up risk if poured out | Typically part of a tablet counting machine plus counting and bottling line |
| Sachets / stick packs | Powders, granules, single-serve oral liquids | Dose-by-dose separation; good portability | Travel friendly; portion control | More individual pieces; tear quality matters | Commonly made on a sachet packing machine/ stick packing machine; some products use a premade pouch packing machine |
Other common pharmaceutical packaging types you’ll come across
Even if you mostly notice blisters, bottles, and sachets, you’ve likely encountered several other pharmaceutical packaging types without thinking much about them. These formats exist because certain products need a different kind of protection, a different user experience, or a different route of administration.
Strip packs are often confused with blisters because they also present medicines in dose-by-dose strips. The difference is structural: blisters form cavities (often in plastic or foil), while strip packs typically sandwich tablets between layers of film/foil and seal around each dose. Strip packaging can be useful when you want a slim, lightweight format and a strong barrier, but it’s not ideal for every tablet shape or handling scenario.
Unit-dose and calendar packs are blister-like formats designed around adherence and safety. Hospitals and care settings often use unit-dose dispensing because each dose can be tracked, labeled, and handled with fewer “open container” steps. Calendar packs—where doses are arranged by day and time—reduce confusion for people taking multiple medications, and they make missed doses obvious at a glance.
Tubes are most common for topical medicines such as creams, ointments, and gels. The tube protects the product from contamination and makes it easy to dispense a controlled amount, but it also brings its own challenges: headspace, backflow contamination risk, and the need for closures that don’t leak or dry out.
Vials and ampoules are core formats for injectables. Vials are typically multi-dose or single-dose containers sealed with stoppers and caps, while ampoules are usually sealed glass containers opened by snapping the neck. Both formats exist because sterile products require extremely tight microbial protection and compatibility with the drug over time.
Prefilled syringes combine container and delivery device in one. They’re widely used where ease of use, dosing accuracy, and reduced preparation steps matter. Because they are both package and device, they often involve additional protective components (needle shields, rigid trays, secondary overwraps) to keep them clean, secure, and stable.
Pouches are increasingly common for supplements and some OTC categories. They’re not always “pharma” in the strict regulatory sense, but they live adjacent to it—especially for powders, gummies, and multi-serve products. Stand-up pouches can reduce shipping volume and improve shelf presence, while single-serve pouches and stick packs compete directly with sachets for portability.
To make the landscape easier to see, here’s a quick map from product type to the packaging forms people most often encounter.
Table 2. Quick map: dosage form → common packaging formats
| Dosage form | Packaging formats people commonly see |
| Tablets / capsules | Blister packs, bottles, strip packs, unit-dose/calendar packs |
| Powders / granules | Sachets, stick packs, pouches, sometimes bottles with scoops |
| Oral liquids | Bottles, unit-dose cups, sachets/sticks for single-serve liquids |
| Topicals (creams/gels) | Tubes, pumps, jars (less common for regulated drugs) |
| Injectables | Vials, ampoules, prefilled syringes (often with protective trays/overwraps) |
What each package protects against: moisture, light, oxygen, and contamination
Most of the “why” behind pharmaceutical packaging types comes down to a few stressors that degrade drugs over time. Moisture is a major one: many tablets and capsules can soften, crack, dissolve poorly, or lose potency faster when repeatedly exposed to humid air. That’s why you’ll see blister packs for moisture-sensitive products, and why bottle formats often pair with a desiccant and a closure designed to reduce moisture ingress. Light is another stressor. If a drug is light-sensitive, the packaging may use opaque materials, tinted bottles, or foil layers that block UV and visible light.
Oxygen can also be a problem, especially for certain vitamins and sensitive actives. Packaging that reduces oxygen exposure—through barrier materials, tight seals, or protective atmospheres—helps slow oxidation. And finally, contamination control is critical: some products need to prevent direct contact with hands, reduce microbial exposure, or remain sterile (injectables). That’s where vials, ampoules, prefilled syringes, and certain unit-dose formats earn their place.
Safety and labeling: lot numbers, expiry dates, and tamper evidence
Another reason packaging differs is simple human factors. People make mistakes under stress, in low light, or when juggling multiple medications. Packaging that makes doses visible (blisters), labels clear (cartons), and tamper evidence obvious can reduce certain types of errors.
Many users first notice packaging safety through tamper-evident features: sealed cartons, shrink bands, induction seals, tear notches, or blister designs that show clear damage when opened. These features help people trust what they’re taking and spot obvious problems before use. Lot numbers and expiry dates also matter more than most people realize, because they help with recalls, pharmacy verification, and safe storage. Depending on the product, this information may be printed on the carton, the blister foil, the bottle label, or the sachet itself.
This is where secondary packaging quietly does a lot of work. The carton and leaflet can carry dosage instructions, warnings, language requirements, and traceability codes. In production, those cartons are typically handled and assembled on a cartoning machine, which is one reason cartons can look very consistent and information-dense without being cluttered.
Sustainability: what’s easy to recycle (and what isn’t)
Sustainability is now part of the conversation around pharmaceutical packaging types, but it’s also an area where expectations and reality can diverge. Some packages are technically recyclable but not accepted in many municipal systems. Others are difficult because they combine multiple layers (for example, plastic + foil) that are hard to separate. Even when materials are recyclable in principle, contamination concerns and collection infrastructure determine what happens in practice.
That said, the direction of travel is clear: more interest in materials that reduce mixed-layer complexity, more pressure to reduce packaging volume, and more experimentation with designs that keep drug protection high while improving end-of-life options. For regulated medicines, though, protection and safety come first; packaging changes tend to be careful and evidence-driven because stability and patient safety are non-negotiable.
Behind the pack: how these formats are made (and where the machines fit)
It’s useful to understand that most packaging formats follow a repeatable “protect, portion, and label” logic—just executed differently depending on the product. Blister packs are typically made by forming cavities, placing tablets or capsules, sealing with lidding foil, printing key information, and cutting the final cards. Bottling lines generally handle bottle feeding/unscrambling, counting or filling, optional desiccant insertion, capping, labeling, and final cartoning or bundling. Sachet and stick formats dose a powder or liquid, form or open the pack, seal it, then cut or discharge finished units.
If you manage a brand or operate a production site, this is where packaging and equipment intersect. A facility may describe its setup broadly as pharmaceutical packaging equipment, pharmaceutical packaging machinery, or simply a packaging machine for pharmaceuticals, but the reality is usually a linked line of specialized stations. At Ruidapacking, the most common “everyday” formats in this article are typically supported by dedicated blister packing machine, capsule counting machine, table counter, and sachet packing machine for single-serve products—each chosen to match the product’s stability needs and the end user’s handling experience.
Wniosek
Packaging is easy to overlook until it causes friction—when a blister is hard to open, a bottle clumps from humidity, or a sachet tears poorly. But in most cases, pharmaceutical packaging types exist for practical reasons: protecting the drug, controlling the dose, and helping people use it safely. Once you know what each format is “trying to solve,” the variety becomes less confusing and more logical. Blisters emphasize dose separation and protection, bottles emphasize convenience for larger counts, sachets emphasize portability and single-serve control, and the other formats exist because some products need sterility, device integration, or specialized dispensing.
FAQs
1) What is a blister pack?
A blister pack is a package where each dose sits in its own sealed cavity, usually covered by a foil layer. You access the dose by pushing through or peeling back, depending on the design.
2) Blister packs vs bottles: which is better for moisture?
It depends on the specific materials and closure design, but dose-by-dose sealing can reduce repeated exposure for the remaining doses. Bottles often rely on closures and desiccants to manage moisture after opening.
3) What’s the difference between blister packs and strip packs?
Blisters typically form a cavity that holds the dose. Strip packs usually seal doses between layers of flexible material without forming a rigid cavity.
4) Can blister packs be recycled?
Some are difficult to recycle because they combine foil and plastic layers. Local recycling rules vary, and many systems don’t accept mixed-material packs.
5) What is unit-dose packaging?
Unit-dose packaging separates medication into individually packaged doses, commonly used in hospitals and care settings for safer handling and tracking.
6) Why are some blister packs hard to open?
Designs often balance safety (including child resistance), barrier performance, and durability during shipping. Some packs prioritize safety over easy push-through.
7) Where can I find the expiry date or lot number?
It may be printed on the carton, the blister foil, the bottle label, or the sachet itself. If you can’t find it, check both the outer carton and the primary pack.
Referencje
U.S. Food and Drug Administration (FDA): Drug labeling, packaging, and tamper-evident concepts (consumer-facing guidance and regulatory frameworks).
World Health Organization (WHO): Good manufacturing practice (GMP) principles relevant to packaging operations.
International Council for Harmonisation (ICH): Stability guidance (e.g., ICH Q1 series) that underpins why barrier and protection matter.

